Lewis dot draw structures diagrams easy made
Table of Contents
Table of Contents
Have you ever been stuck trying to draw a Lewis dot diagram for a chemistry assignment, only to end up frustrated and confused? If so, you’re not alone. Many students struggle with this task, but with a little bit of guidance, it can become much easier.
The Struggle of Drawing a Lewis Dot Diagram
Drawing a Lewis dot diagram can be difficult for a number of reasons. Firstly, it requires you to have a good understanding of electron configurations and valence electrons. Additionally, the diagrams can be complex and confusing, especially for molecules with many atoms. Finally, there are multiple ways to draw the same molecule, which can lead to confusion and uncertainty.
How to Draw a Lewis Dot Diagram
Despite its difficulties, drawing a Lewis dot diagram is an essential skill for any chemistry student. Here’s a step-by-step guide to make the process easier:
- Determine the total number of valence electrons in the molecule.
- Identify the central atom, which is usually the least electronegative element.
- Connect the atoms with single bonds.
- Distribute the remaining electrons as lone pairs on the outer atoms.
- Place any remaining electrons as lone pairs on the central atom.
- If necessary, convert lone pairs into double or triple bonds to achieve an octet for each atom.
It’s important to remember that the Lewis dot diagram is just a model, and it doesn’t necessarily reflect the true geometry of the molecule.
Personal Experience Drawing a Lewis Dot Diagram
When I first started learning how to draw Lewis dot diagrams, I found it to be an overwhelming and confusing task. But after a few attempts and with the help of my professor, I began to understand the process better. One thing that helped me was using a color-coded system to keep track of which electrons belonged to which atoms.
 Tips for Drawing a Lewis Dot Diagram
Tips for Drawing a Lewis Dot Diagram
Here are some additional tips to help make drawing Lewis dot diagrams easier:
- Practice, practice, practice!
- Use a periodic table to determine the number of valence electrons in each atom.
- When in doubt, remember that molecules tend to minimize their potential energy by spreading out their electrons as much as possible.
- If you’re struggling with a particular molecule, try breaking it down into smaller parts and drawing the Lewis dot diagrams for each part individually.
Common Mistakes to avoid when drawing a Lewis Dot Diagram
It’s also important to be aware of common mistakes to avoid when drawing Lewis dot diagrams. These include:
- Forgetting to count all of the valence electrons.
- Placing too many (or too few) lone pairs on an atom.
- Not following the octet rule (or, in some cases, the duet rule).
- Forgetting to include formal charges.
 Example Lewis Dot Diagram
Example Lewis Dot Diagram
Let’s walk through an example to illustrate the process of drawing a Lewis dot diagram. We’ll use the molecule for water (H2O).
- Determine the total number of valence electrons: 6 (for oxygen) + 1 x 2 (for hydrogen) = 8
- Identify the central atom: oxygen
- Connect the atoms with single bonds: O-H-H
- Distribute the remaining electrons as lone pairs on the outer atoms: H: 2; O: 4
- Place any remaining electrons as lone pairs on the central atom: O: 2
The final Lewis dot diagram for water would look like this:
 Question and Answer
Question and Answer
Q: Can a single atom have a Lewis dot diagram?
A: Yes, a single atom can have a Lewis dot diagram. The number of valence electrons for the atom will be the same as the group number on the periodic table.
Q: What happens if there are too few electrons to meet the octet rule?
A: In some cases, molecules may not be able to achieve an octet for every atom. For example, in diatomic molecules like N2 and O2, each atom shares its electrons to achieve a stable configuration with just two electrons each.
Q: Can more than one resonance structure be drawn for a molecule?
A: Yes, some molecules may have multiple resonance structures that can be drawn. These structures will have the same placement of atoms, but different arrangements of electrons.
Q: What is a formal charge?
A: A formal charge is a way of keeping track of the number of electrons an atom “owns” in a Lewis dot diagram. It is calculated by subtracting half the number of shared electrons and all of the lone electrons from the total number of valence electrons for the atom.
Conclusion of How to Draw a Lewis Dot Diagram
While drawing Lewis dot diagrams can be challenging, with practice and persistence, you can master the skill. Remember to take it step by step, and to refer back to the periodic table and other resources as needed. Keep practicing, and soon you’ll be drawing these diagrams with ease.
Gallery
LEWIS DOT DIAGRAM - Unmasa Dalha

Photo Credit by: bing.com / dot lewis diagram structures diagrams chemistry bohr rutherford
Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures - YouTube
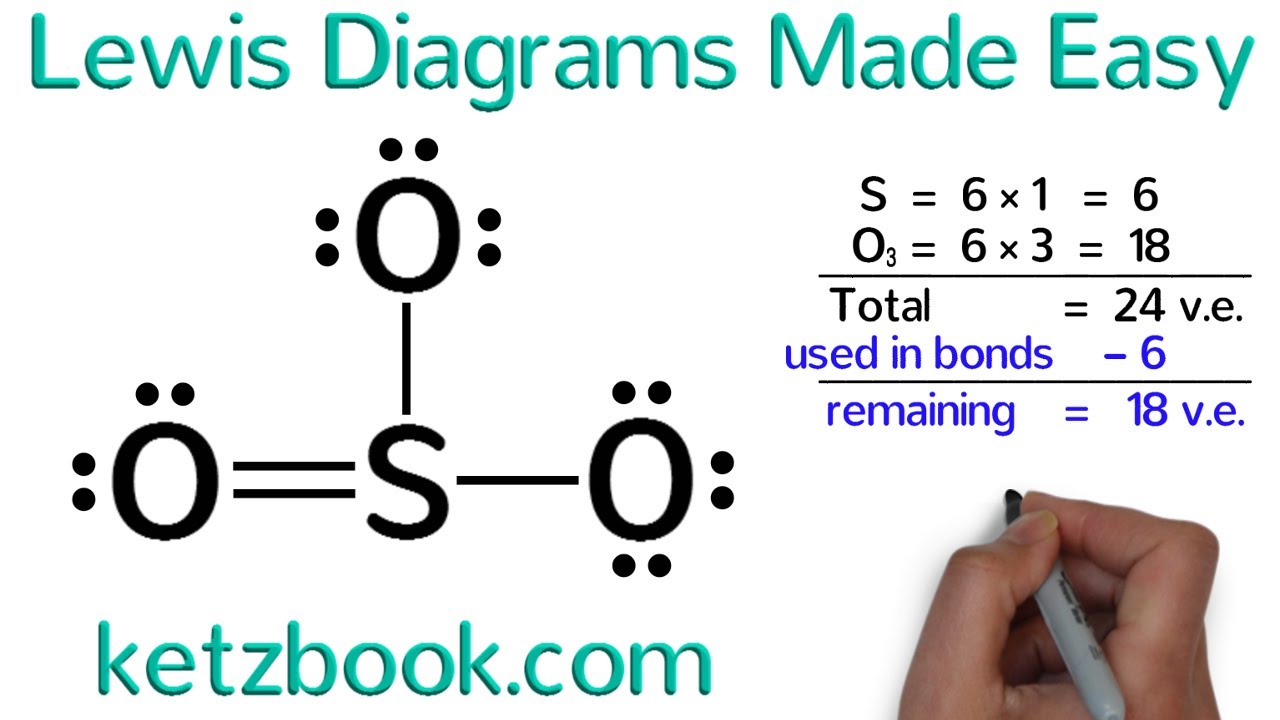
Photo Credit by: bing.com / lewis dot draw structures diagrams easy made
How To Draw A Lewis Dot Diagram - Derslatnaback
Photo Credit by: bing.com / lewis diagram dot draw nf3 wiring schematic
How To Draw Lewis Dot Structure
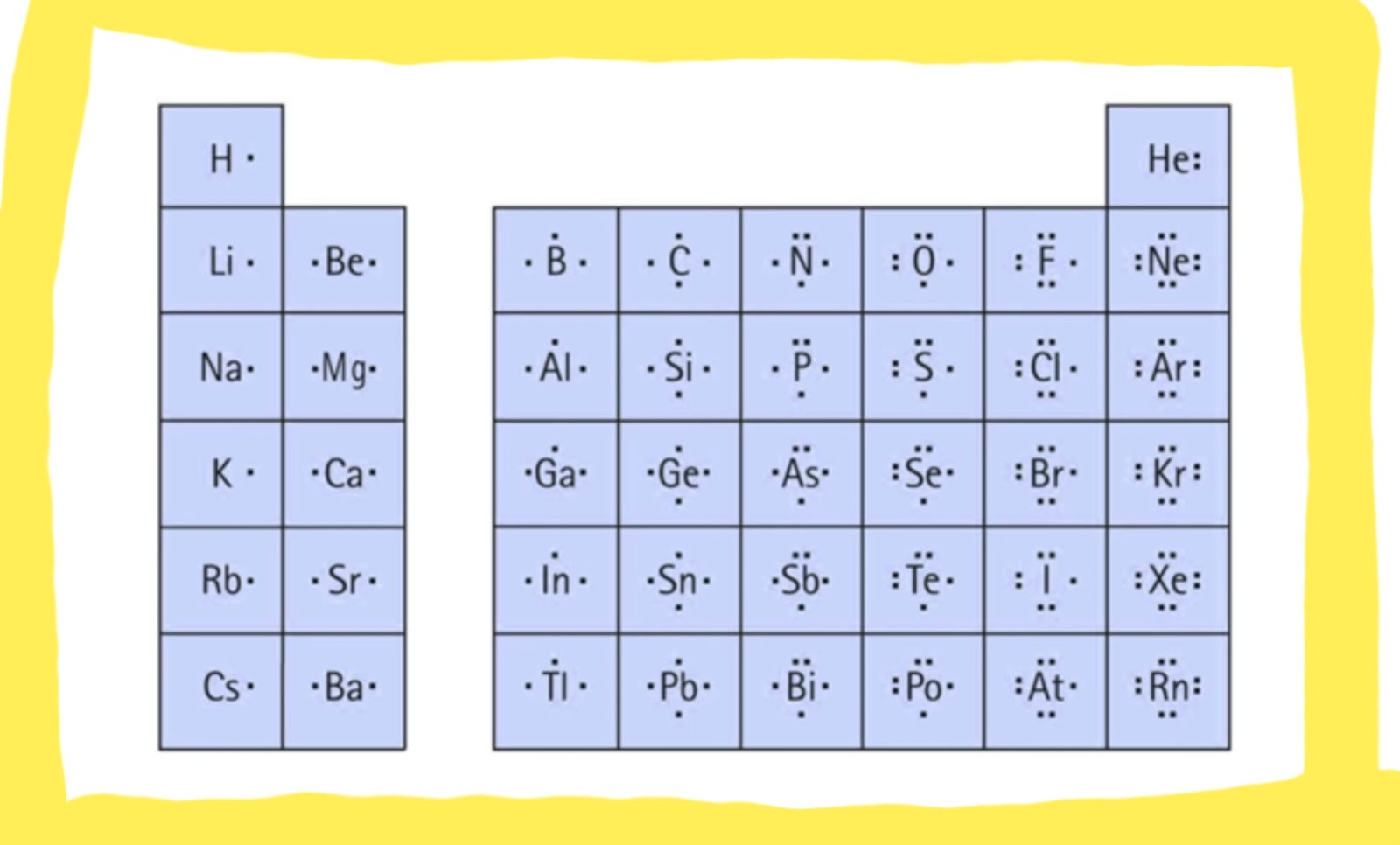
Photo Credit by: bing.com / lewis dot structure structures diagram valence draw electrons drawing represent often used number
Lewis Dot Diagram Practice

Photo Credit by: bing.com / studylib s2 skeletal